You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Graphic Thread
- Thread starter Glen
- Start date
Alex Richards
Donor
Interesting, though the fact that you'd have a very odd irregular increase in valence electron count and atomic mass after the 3rd column* makes me wonder about it's long term survival. At some point it just feels someone's going to say 'why don't we just put the Alkali Earths and Metals at the top so that we go from 1 electron and count upwards?' and you've effectively just rotated the OTL table by 90 degrees and mirrored it.
Though even that would still be interesting.
*Going roughly 1, 2, 18 and then decreasing to 3 from there.
Though even that would still be interesting.
*Going roughly 1, 2, 18 and then decreasing to 3 from there.
There doesn't appear to be a general thread for posting artwork so let's start one.
I'll start us off with my alternate Periodic Table from the DSA timeline...

Really interesting idea! How are the atoms sorted?
Glen
Moderator
Interesting, though the fact that you'd have a very odd irregular increase in valence electron count and atomic mass after the 3rd column* makes me wonder about it's long term survival.
*Going roughly 1, 2, 18 and then decreasing to 3 from there.
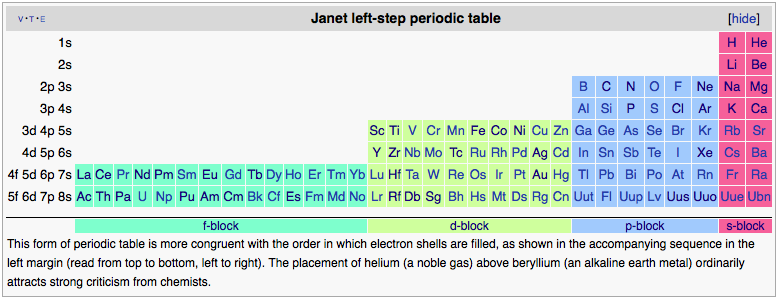
I don't quite know what you're indicating with the 1, 2, 18 comment. Basically it is a flipped and rotated version of the Janet Periodic Table with the 1s & 2s on the same column rather than separate.
At some point it just feels someone's going to say 'why don't we just put the Alkali Earths and Metals at the top so that we go from 1 electron and count upwards?'
Because it's less compact of a presentation graphically, and I'm not certain that the 1 electron count up is that important. Could be just me but...
and you've effectively just rotated the OTL table by 90 degrees and mirrored it.
Though even that would still be interesting.
Oh yeah?
Glen
Moderator
I don't quite know what you're indicating with the 1, 2, 18 comment. Basically it is a flipped and rotated version of the Janet Periodic Table with the 1s & 2s on the same column rather than separate.
Ah, I see - you're referring to group number - but those group number schemes wouldn't be the same in the ATL.

Because it's less compact of a presentation graphically, and I'm not certain that the 1 electron count up is that important. Could be just me but...[/QUOTE]
To elaborate, the Janet Alternate isn't based on valence electron numbers but rather electron shells. If you want to think of it another way, the bottom of my stairway is focused on the 'valence' electrons, but quasi centered on the Noble Gases so that the elements that need to have an electron added and those that need to have an electron lost to balance their shells flank the filled shell noble gases.
Alex Richards
Donor
I don't quite know what you're indicating with the 1, 2, 18 comment. Basically it is a flipped and rotated version of the Janet Periodic Table with the 1s & 2s on the same column rather than separate.
Well, there's several reasons that one attracts strong criticisms from Chemists.
But basically, if you look at the 4th column, then the number of electrons in the valence shell (which determines the properties in great deal) will count up from 3 (Coraxium) to 18 (Oblium) then drop down to 1 and go up to 2. This by itself is perhaps not too much of an issue, but this also means that you increase in mass down the column, until the last two which are suddenly lighter in mass than the ones at the top.
Suffice to say this makes it rather harder to read off trends in the properties of atomic weight and so forth- something compounded by having Hydrogen in the same period as the Halogens which is... yeah not exactly accurate.
It actually raises a rather serious question about how you even come to this as the standard table in the 30s- the Janet table relies on a quantum understanding of electron orbital filling for its reasoning, whereas electron count with a couple of exceptions follows atomic mass- indeed the table was actually ordered based on that, but with a couple of elements transposed to ensure properties were the same down the column (Chlorine in particular IIRC).
Unless this is a much later table that supercedes an earlier one, I'm struggling to see how you'd come up with this arrangement first.
EDIT: Oh god your reasoning is a little out- the valence electrons are not the s shell, it's the outermost partially filled shell. For Aluminium, this includes both the s and p orbitals, for Iron it's the s and d orbitals, and it's definitely not accurate to state that the s shell is filled after the d shell as there's some complicated electron shell filling patterns going on which basically mean that for a couple of configurations you end up with the s-shell electrons being transferred to the d orbitals but otherwise you have a filled s shell but partially filled d shell.
Glen
Moderator
Really interesting idea! How are the atoms sorted?
Mostly by traditional methods but with an emphasis on the electron shells, with the exception of Hydrogen and Helium being placed right above the groups they more closely resemble to satisfy the physical chemists.
Glen
Moderator
Well, there's several reasons that one attracts strong criticisms from Chemists.
Other than Hydrogen & Helium? (for which you'll note my version makes an allowance)
But basically, if you look at the 4th column, then the number of electrons in the valence shell (which determines the properties in great deal) will count up from 3 (Coraxium) to 18 (Oblium) then drop down to 1 and go up to 2. This by itself is perhaps not too much of an issue, but this also means that you increase in mass down the column, until the last two which are suddenly lighter in mass than the ones at the top.[/QUOTE]
Take another look at the alignment - they don't go lighter - 1 & 2 are shifted one higher than in the traditional periodic table - which is fine, since it's really a spiral anyway.
Suffice to say this makes it rather harder to read off trends in the properties of atomic weight and so forth- something compounded by having Hydrogen in the same period as the Halogens which is... yeah not exactly accurate.
There is no place that is exactly accurate for Hydrogen. I could make arguments for it's traditional location, for where I have placed it, and for above Carbon.
It actually raises a rather serious question about how you even come to this as the standard table in the 30s- the Janet table relies on a quantum understanding of electron orbital filling for its reasoning, whereas electron count with a couple of exceptions follows atomic mass- indeed the table was actually ordered based on that, but with a couple of elements transposed to ensure properties were the same down the column (Chlorine in particular IIRC).
Unless this is a much later table that supercedes an earlier one, I'm struggling to see how you'd come up with this arrangement first.
This is a much later table, and it is an attempt at compromise between chemists and physicists.
EDIT: Oh god your reasoning is a little out- the valence electrons are not the s shell, it's the outermost partially filled shell.
I actually do know that - if I had been ordering it by s shell, it would have looked very different.
For Aluminium, this includes both the s and p orbitals,
Hmmm, didn't know that one.
for Iron it's the s and d orbitals, and it's definitely not accurate to state that the s shell is filled after the d shell as there's some complicated electron shell filling patterns going on which basically mean that for a couple of configurations you end up with the s-shell electrons being transferred to the d orbitals but otherwise you have a filled s shell but partially filled d shell.
Which is part of why I don't believe that they reference valence number for the transitional metals.
Alex Richards
Donor
Other than Hydrogen & Helium? (for which you'll note my version makes an allowance)
Not sure on all the matters here, I'm a chemist myself but not an expert on this here.
Ah, slightly misread that yes. I'd still consider it to be problematic, mainly because the transition metals of the 3d orbital do actually involve the 4s orbital in a lot of interactions and the 5s basically doesn't come into it.Take another look at the alignment - they don't go lighter - 1 & 2 are shifted one higher than in the traditional periodic table - which is fine, since it's really a spiral anyway.
True indeed, it's really why I tend to prefer the 'floating at the top of the table' layout as it doesn't make sense to try and force it into one column or another.There is no place that is exactly accurate for Hydrogen. I could make arguments for it's traditional location, for where I have placed it, and for above Carbon.
Well, that makes more sense, not that the physicists really do much with the periodic table outside the radioactive isotopes anyway.This is a much later table, and it is an attempt at compromise between chemists and physicists.
Yeah, it's actually a really interesting process where the covalent bonding process for aluminium (and carbon, nitrogen and oxygen for that matter) involves a hybridisation of the s and p orbitals to form a set of identical sp orbitals which can then have identical overlap rather than the different shapes of the s and p orbitals. A lot of this comes down to molecular symmetry.Hmmm, didn't know that one.
Not strictly accurate, the blocks are still ordered by valence electrons (so you'll hear people talking about group 3 Transitional Metals, or group 5 metals), but the normal rules for electron shell filling are suspended for the transition metals and it's just sort of assumed that the d block doesn't get involved when you get to groups 13-18.Which is part of why I don't believe that they reference valence number for the transitional metals.
shiftygiant
Gone Fishin'
Cool, somewhere I can dump all my crap:

For a Timeline that will never happen.

Which I made as a joke.


And you can find context here!
/i did graphic design for four years


For a Timeline that will never happen.

Which I made as a joke.


And you can find context here!
/i did graphic design for four years
Glen
Moderator
Cool, somewhere I can dump all my crap:
View attachment 272582
For a Timeline that will never happen.

Which I made as a joke.


And you can find context here!
/i did graphic design for four years
Fun stuff there - u especially like the Hamilton one.
Tallest Skil
Banned
So stuff like this? Campaign poster for Lincoln’s reelection in The United States of Ameriwank.

On that note, anyone heard from Big Tex? Been almost a year since the last time he posted, which was about an attempted account invasion. Hope he’s okay... Haven’t heard from him.

On that note, anyone heard from Big Tex? Been almost a year since the last time he posted, which was about an attempted account invasion. Hope he’s okay... Haven’t heard from him.
Glen
Moderator
So stuff like this? Campaign poster for Lincoln’s reelection in The United States of Ameriwank.

On that note, anyone heard from Big Tex? Been almost a year since the last time he posted, which was about an attempted account invasion. Hope he’s okay... Haven’t heard from him.
Yep things like that - very nice!
Well, I'm not sure if this qualifies for this thread either, but I designed a newsline/ticker for アメリカ放送協會 (Amerika Hōsō Kyōkai, American Broadcasting Company), the state-run media for the Pacific States of America (a little bit more info here) for my Man in the High Castle timeline, and I wanted to crosspost it here. Translations for everything are below.

Translations (left to right, top to bottom)
1)
Title: San Francisco: protestors calling for "democracy now!"
Ticker: ...okyo and Kyoto, the general strike continues · Leader of the All-Pacific Federation of Students: "Vietn...
2)
Title: Xinjiang: Uyghur group announces "We demand a state for the Uyghurs"
Ticker: ...leader of Occupy Central at Hiiragi University: "The New Leaf Movement is a student movement for democr...
3)
Title: In Singapore, the police line is failing as officers break ranks
Ticker: ...The president of Malaya says "we will not shoot on our own people" · The parlia...
4)
Title: Over 1000 rail workers block the entrance to Shinjuku Station
Ticker: ...hinzo Abe: "The Japanese Empire has the greatest people, emperor and country in th...
5)
Title: Korean Teachers' Association: We refuse to teach the falsehoods in the Japanese textbooks
Ticker: ...representative said that legalization of the Korean language "is an absolute necessity"...
6)
Title: Governor of Idaho resigns, announces free elections
Ticker: ...oshihiro Fukuyama's announcement: an election is not occurring, please do not att...
7)
Title: Protest in Seattle draws over 30,000 people
Ticker: ...overnor of Washington has announced his resignation effective Wednesday · "He's not going anywh...
8)
Title: Kaeri announces: Elections in Japan and the Pacific States are of the highest importance
Ticker: ...slogan "Occupy Central with Love and Peace" · In English, they are calling themselves "New Leaf Movement"...
9)
Title: "Vietnam is better without Nyugen and with a new leader"
Ticker: ...President Tominaka Gen (Nguyễn Phú Trọng) has not yet made any announcement, but is expected to...
10)
Title: Kaeri's priority: "We are going to occupy Center City until Fukuyama's resignation"
Ticker: ...s in Sacramento · Sympathizers of the general strike are collecting in central Los Angeles for...

Translations (left to right, top to bottom)
1)
Title: San Francisco: protestors calling for "democracy now!"
Ticker: ...okyo and Kyoto, the general strike continues · Leader of the All-Pacific Federation of Students: "Vietn...
2)
Title: Xinjiang: Uyghur group announces "We demand a state for the Uyghurs"
Ticker: ...leader of Occupy Central at Hiiragi University: "The New Leaf Movement is a student movement for democr...
3)
Title: In Singapore, the police line is failing as officers break ranks
Ticker: ...The president of Malaya says "we will not shoot on our own people" · The parlia...
4)
Title: Over 1000 rail workers block the entrance to Shinjuku Station
Ticker: ...hinzo Abe: "The Japanese Empire has the greatest people, emperor and country in th...
5)
Title: Korean Teachers' Association: We refuse to teach the falsehoods in the Japanese textbooks
Ticker: ...representative said that legalization of the Korean language "is an absolute necessity"...
6)
Title: Governor of Idaho resigns, announces free elections
Ticker: ...oshihiro Fukuyama's announcement: an election is not occurring, please do not att...
7)
Title: Protest in Seattle draws over 30,000 people
Ticker: ...overnor of Washington has announced his resignation effective Wednesday · "He's not going anywh...
8)
Title: Kaeri announces: Elections in Japan and the Pacific States are of the highest importance
Ticker: ...slogan "Occupy Central with Love and Peace" · In English, they are calling themselves "New Leaf Movement"...
9)
Title: "Vietnam is better without Nyugen and with a new leader"
Ticker: ...President Tominaka Gen (Nguyễn Phú Trọng) has not yet made any announcement, but is expected to...
10)
Title: Kaeri's priority: "We are going to occupy Center City until Fukuyama's resignation"
Ticker: ...s in Sacramento · Sympathizers of the general strike are collecting in central Los Angeles for...
Last edited:
Hapsburg
Banned
Some of the things I'm posting here would've been cross-posted in the Photos form Alternate Worlds page. But being that they're mostly not-photos, they're probably better fit here.
Portrait of General Alexander Julius Vox, 1st Prince of Tycho. Better known as Xander Vox, Director-General of Republic Security. He is a high-level civil servant holding the simultaneous pay and rank of a Grand Marshal in the Security Service, a Senior Chief of Police, and an Undersecretary of State Security. His personal relationship with Emperor Maximilian led to his appointment as head of the Imperial Guard, which developed into a senior political appointment to head various government departments tasked with VIP security protection and then with the secret police apparatus after Maximilian set aside his imperial titles and was elected Supreme Chancellor of the Republic. In the aftermath of the disorganised response of local police forces to the Pirate War in 3285, he arranged the absorption of all police agencies into his Republic Security Department. His name became associated in the minds of dissenters with terror and repression--though those loyal to the New Order saw him as a crusader for stern justice and order. His affinities are also closely aligned with the Rycon princely family, being that his father was a lesser prince of the Lunarian nobles; he pushed for recognition of his paternity and was given a noble title and estates on Luna in the waning days of the Terran Empire.
In truth, however, Vox is a sadistic psychopath, much like his rival in State Security, General William J. McGrady. Before his political career, he was a brutal mercenary alongside his half-brother Brian Kessar--who joined State Sec as well--and then was a brutal police enforcer. He ruthlessly carries out Maximilian's will purely because it gains him more power.

Portrait of General Alexander Julius Vox, 1st Prince of Tycho. Better known as Xander Vox, Director-General of Republic Security. He is a high-level civil servant holding the simultaneous pay and rank of a Grand Marshal in the Security Service, a Senior Chief of Police, and an Undersecretary of State Security. His personal relationship with Emperor Maximilian led to his appointment as head of the Imperial Guard, which developed into a senior political appointment to head various government departments tasked with VIP security protection and then with the secret police apparatus after Maximilian set aside his imperial titles and was elected Supreme Chancellor of the Republic. In the aftermath of the disorganised response of local police forces to the Pirate War in 3285, he arranged the absorption of all police agencies into his Republic Security Department. His name became associated in the minds of dissenters with terror and repression--though those loyal to the New Order saw him as a crusader for stern justice and order. His affinities are also closely aligned with the Rycon princely family, being that his father was a lesser prince of the Lunarian nobles; he pushed for recognition of his paternity and was given a noble title and estates on Luna in the waning days of the Terran Empire.
In truth, however, Vox is a sadistic psychopath, much like his rival in State Security, General William J. McGrady. Before his political career, he was a brutal mercenary alongside his half-brother Brian Kessar--who joined State Sec as well--and then was a brutal police enforcer. He ruthlessly carries out Maximilian's will purely because it gains him more power.
The logo of the "Nixonite-Reaganite" party of the Silent and Moral Majority.
Appropriately enough, it's called the Majority Party.
The motto is obviously a compromise between the Silent and Moral parts.

Appropriately enough, it's called the Majority Party.
The motto is obviously a compromise between the Silent and Moral parts.
shiftygiant
Gone Fishin'
There doesn't appear to be a general thread for posting artwork so let's start one.
I'll start us off with my alternate Periodic Table from the DSA timeline...

Logically, 105 (below Washingtonium), OTL named Dubnium and by the Mendeleev naming scheme "Eka-Washingtonium", would need to be Xashingtonium because the predicted elements are always one letter ahead in the decisive letter.
And 107 would be Movecraftium, and 117 Rurpurium.
I love such alternate periodic tables! One of them, which I have also used in a mapgame, is on my German Wikipedia user page (http://de.wikipedia.org/wiki/Benutzer:ObersterGenosse!
Share:


